- TOP
- Mission & Works
Repertoire Genesis Inc.
Mission Statement:
“Curing the Incurable”
We deliver effective and efficient new therapeutic and diagnostic methods to patients worldwide using our unique “immune-diversity analysis technology” and our optimal partnerships with academia and pharmaceutical companies.
The immune system is highly beneficial in protecting our bodies from disease by recognizing and attacking pathogens, cancer cells, and other abnormal cells. However, the immune system also has adverse effects such as attacking one’s cells (autoimmune diseases) and overreacting to substances usually harmless to the body (allergies).
The immune system is one of nature’s most sophisticated diagnostic and therapeutic mechanisms. However, because the immune system is not yet fully understood, its potential has not been fully utilized in the medical field.
Thus, we are convinced that if we can understand the complexity of the immune system, we could develop new therapeutic and diagnostic methods for various diseases such as cancer, autoimmune diseases, and infectious diseases.
Core Technology: The TCR/BCR Repertoire Analysis
Although there have been attempts to develop new therapeutic and diagnostic methods based on a detailed understanding of the immune system, it has not been easy to achieve. This is because it is technically difficult to accurately grasp the entire mechanism of the immune system due to its enormous diversity.
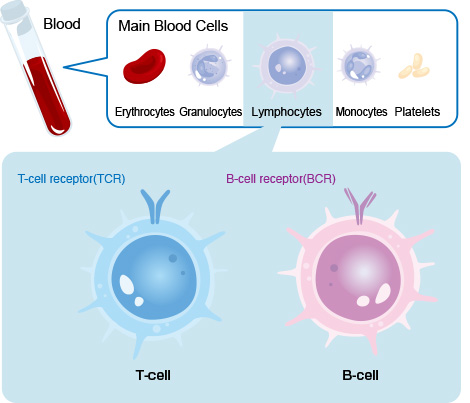
T and B cells in blood
T-cell receptors (TCR) and B-cell receptors (BCR)
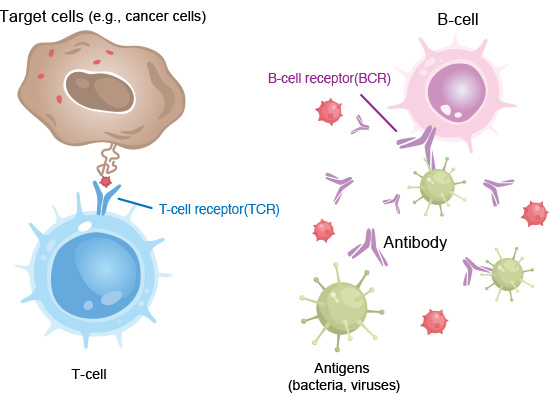
We have developed a next-generation “TCR/BCR Repertoire Analysis,” which is essential for understanding the diversity and specificity of the immune system.
The TCR/BCR Repertoire Analysis developed by our company can clarify what kind of antigen individual T and B cells respond to (specificity) and how diverse their receptors are as a whole (diversity) by comprehensively analyzing the receptors held by T and B cells.
Our body is equipped with an immune system that defends it against pathogens such as bacteria and viruses from invasion. T and B cells play the most essential roles in the immune system.
T-cell receptors (TCRs) and B-cell receptors (BCRs) are responsible for recognizing and attacking antigens of various pathogens, as well as for detecting and eliminating abnormal cells that arise in the body, such as cancer cells.
For this reason, these cells have different receptor molecules on their surface to recognize other antigens depending on their receptor type.
Expansion of our Immune-Diversity Analysis Technology for Medical Development

Since its establishment in October 2014, our company has conducted analyses in many disease fields such as cancer, infectious diseases, and autoimmune diseases, focusing on TCR/BCR Repertoire Analysis, which is a detailed genetic analysis of the major lymphocyte TCR and BCR. We have characterized over one billion T-cell and B-cell receptor genes from over 10,000 samples and have established collaborations and partnerships with over 170 academic institutions and 30 pharmaceutical companies.
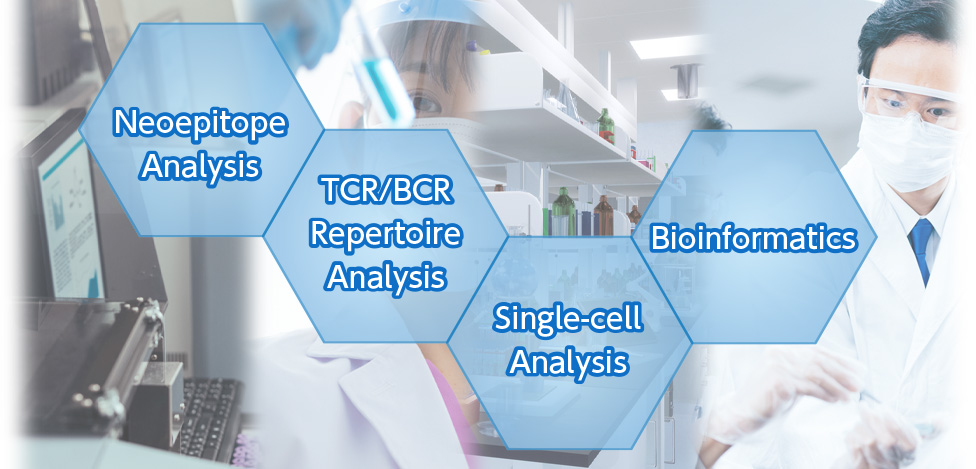
Additionally, by combining the results of these analyses with our advanced bioinformatics technology and the vast amount of data we have accumulated, we can provide high-value-added solutions for customer’s medical research and clinical development purposes and issues.
In addition to the TCR/BCR Repertoire Analysis, we are also developing various technologies for immune-diversity analysis as multifaceted technologies are required to elucidate the immune system and develop new therapeutic and diagnostic methods. For instance, we developed the neoepitope analysis technology that can accurately determine antigens (neoepitopes) expressed only in diseased cells, which differ from normal cells.
Additionally, by combining “single-cell analysis” and TCR/BCR Repertoire Analysis, we can determine T and B cells that cause immune responses on a single cell basis, obtain their TCR/BCR sequences, and promote applied research and development such as the development of cell therapy using “genome editing and gene transfer technologies.”
In this way, our company’s immune-diversity analysis technology is centered on its core technology, the TCR/BCR Repertoire Analysis, and is greatly expanding.
●Bioinformatics technology: This technology integrates life and information sciences and elucidates the biological phenomena by analyzing various “information” of life, such as DNA, RNA, and proteins, using algorithms of information science and statistics.
●Neoepitope analysis technology: Technology for analyzing epitopes that can be recognized by immune cells based on genetic mutations in cancer cells.
●Genome editing technology: A type of genetic engineering in which DNA is inserted, deleted, modified, or replaced in the genome of a living organism.
●Gene transfer technology: This technology introduces a target gene into various cells.
Broad Range of Target Disease Areas and Expanding Business Opportunities
Broad Range of Target Disease Areas
The field of medical development using the immune system, which is our target, is one of the fastest developing markets expected to expand in the future. Using our unique immune-diversity analysis technology, we can contribute to the understanding of many diseases involving the immune system, such as cancer, infectious diseases, autoimmune diseases, allergies, and organ transplantation.
Development of Medical Treatment using the Immune System
(Basic Research, Prevention, Diagnosis, and Treatment)
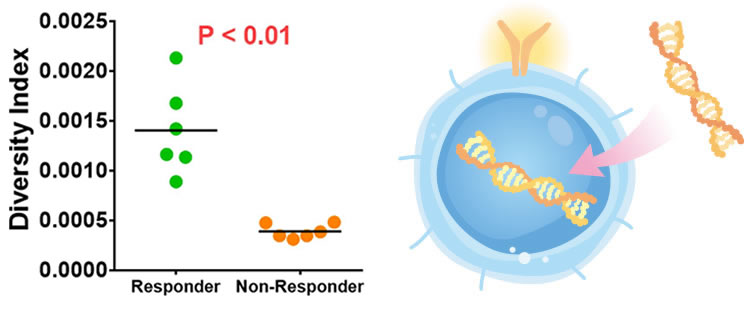
As new coronavirus infections, which will continue to spread after 2019, become a major social issue worldwide, business opportunities to which we can contribute are expanding, including supporting the development of vaccines to prevent infectious diseases and the development of biomarkers to predict the severity of illness caused by an infection.
Recently, the environment for drug discovery has changed dramatically, with pharmaceutical companies entering the diagnostics business and the field of prevention. Our company can find potential biomarkers from the information on receptors of millions of immune cells in patients’ specimens, which can be quickly linked to the development of diagnostic applications. By providing customers with our technologies that apply the immune system’s functions of disease monitoring/early detection/treatment, we expect to significantly expand our business.
●Immune Cell Therapy (TCR-T, CAR-T): A type of cancer immunotherapy in which the genes of T cells are modified to produce special proteins, enabling the modified T cells to recognize and attack cancer cells efficiently.
A Business Model that Achieves both a Stable Revenue Base and High Growth Potential
We work with customers to develop basic and applied medical treatments using our proprietary immune-diversity analysis technologies. This business model enables us to build a stable revenue base while simultaneously achieving high growth potential by offering various value propositions from upstream to downstream of the medical development process.
A Business Model that Achieves both a Stable Revenue Base and High Growth Potential
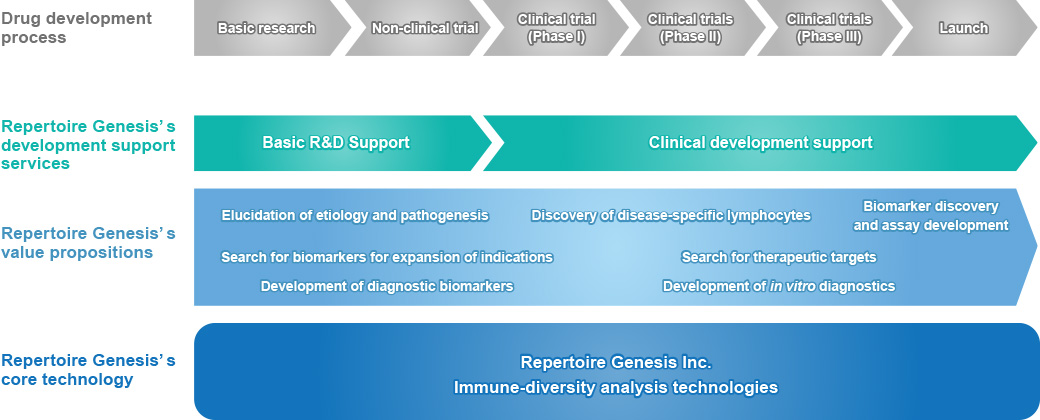
Repertoire Genesis’ One-Source, Multi-Use Strategy
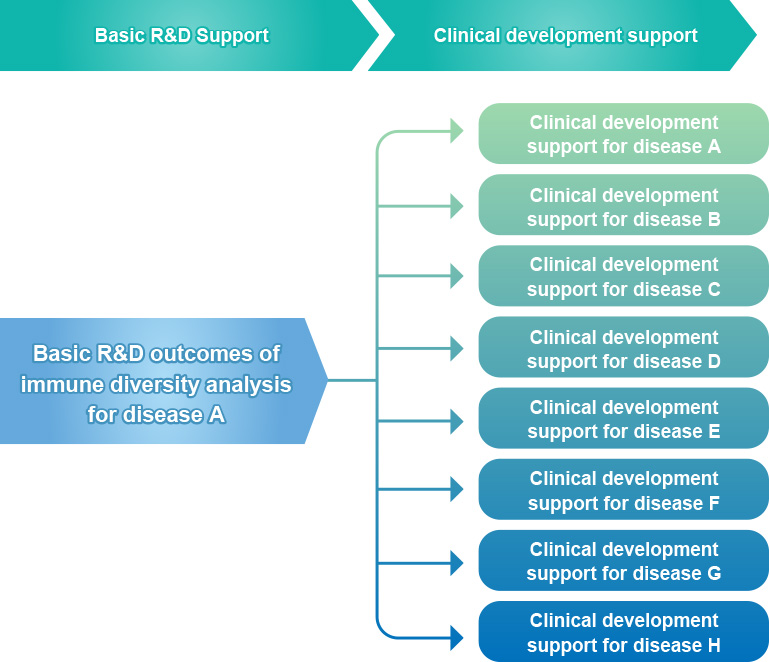
For academics, we support the creation of highly innovative research results by providing our unique “R&D support services” for customer’s basic research needs.
Additionally, when a certain level of result is obtained in basic research, we move from the “basic research phase” in academia to the “clinical development phase” at pharmaceutical companies, leading to further long-term partnerships.
In July 2018, we established a system to provide analysis services developed by us following the Good Laboratory Practice. Furthermore, in March 2019, we became the first company in the world to obtain ISO/IEC 17025:2017 accreditation in the field of repertoire analysis.
With the assurance of international credibility for our main immune-diversity analysis technology, we are actively pursuing long-term clinical development with biopharmaceutical companies in Japan and overseas.
● ISO/IEC 17025:2017: One of the international standards common among nations that are developed by the International Organization for Standardization. It certifies the technical competence of laboratories to conduct specific types of testing and calibration, and includes requirements for quality management and technology.